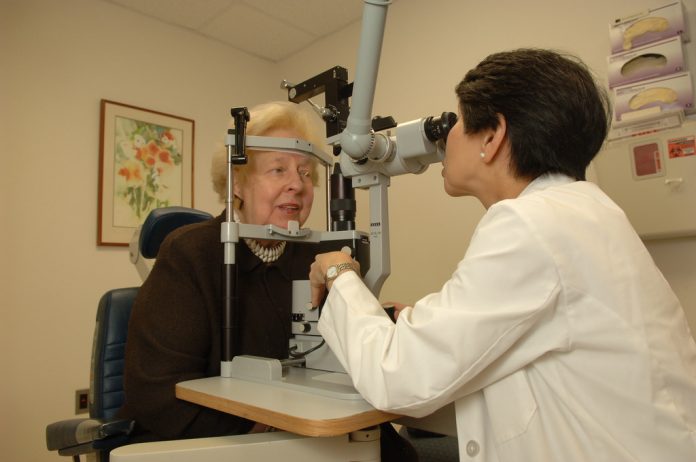
The Food and Drug Administration has approved the sales of IDx-DR, a medical device that employs AI to diagnose eye diseases and other variables only by looking at the patient’s retina without the need of a medical professional. This also marks the first time the FDA approves any AI equipment in history, meaning that science and artificial intelligence are developing at a faster rate than ever.
IDx-DR founder Michael Abràmoff noted the massive addition to the toolshed that this AI could provide to the world of medical science, as it has been proven to be 87 percent accurate on disease diagnosis, which even though it is not fully effective, still remains to be more effective than a doctor. What this means is that people won’t need to be referred to a physician for a test run on vision problems, as AI has stepped into the game.
U.S. FDA approves AI device to detect diabetic eye disease https://t.co/ANVCb02q9W pic.twitter.com/zs794zGRyt
— Reuters Top News (@Reuters) April 11, 2018
IDx-DR: how is it and how does it work?
The medical decision-making software works by analyzing pictures of the patient’s eyes, taken by a special retinal camera Topcon NW400, subsequently uploaded by a nurse or doctor. The first indication is a quality check of the picture since IDx-DR needs a very high image quality to proceed with any diagnostics. As the first picture is analyzed, the software then checks for any basic eye diseases.
The second step consists of a full medical check to discard any possibility of the patient having any signs of diabetic retinopathy, an eye disease in which the excessive amount of sugar obstructs the blood vessels in the back of the eyes. This happens to be the most common eye disease suffered by diabetic individuals, but AI needs to make sure everything is alright with the patient.
FDA permits marketing of the first medical device to use artificial intelligence to detect greater than a mild level of diabetic retinopathy in the eye of adults who have diabetes. https://t.co/diCCIRl6Be #Diabetes #ArtificialIntelligence #AI #MedicalDevice
— FDA/CDRH Industry (@FDAcdrhIndustry) April 11, 2018
Medical AI means more accessible and accurate healthcare for all
The IDx-DR software will then be able to diagnose any other vision problem, but when it was tested on accuracy, the software was submitted to trial with 900 pictures, of which it correctly identified retinopathy 87 percent of the times, while it accurately detected those who did not have retinopathy correctly 90 percent of the time. As this remains to be a rather new technology, it surely has made an impact.
This software stands out for being unique in its autonomous nature. According to Dr Malvina Eydelman, this could prove to be one of the most useful installments in eye medicine, “Early detection of retinopathy is an important part of managing care for the millions of people with diabetes, yet many patients with diabetes are not adequately screened for diabetic retinopathy since about 50% of them do not see their eye doctor on a yearly basis”.
Source: FDA